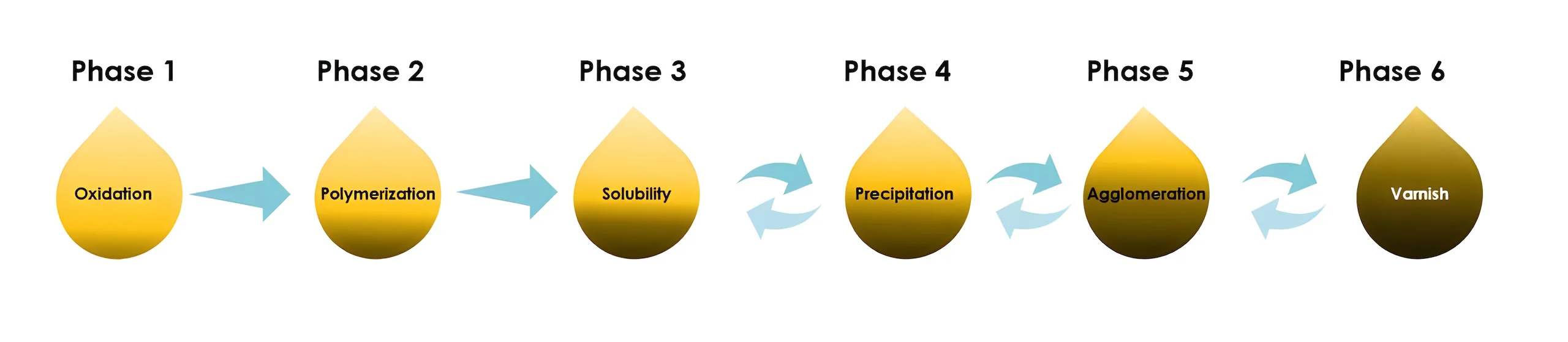
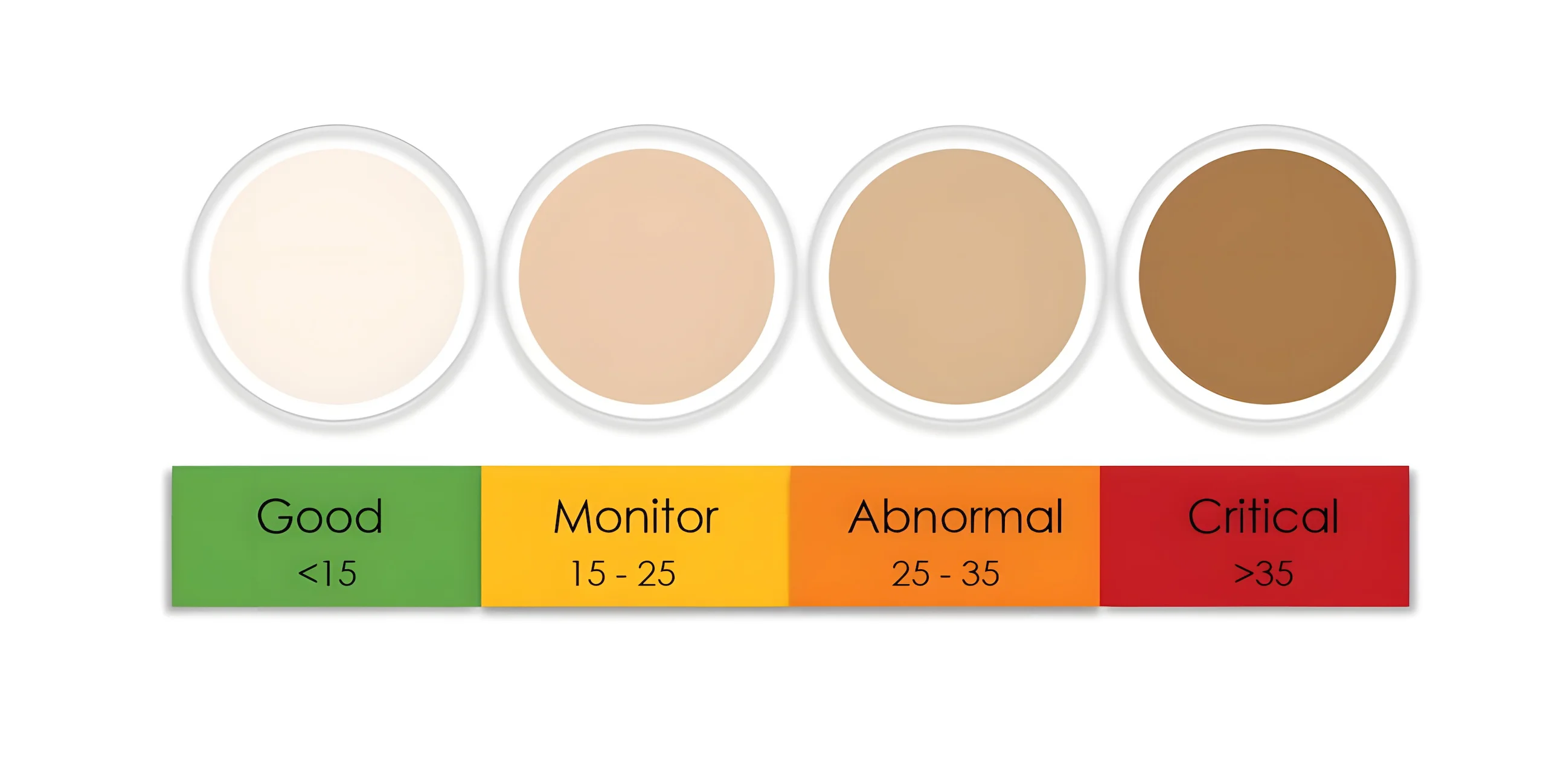
-Chemical: Many chemical reactions occur as the oil ages. Oxidation of oil leads to numerous decomposition products, including insoluble particulates and acids. Heat and the presence of metal particulars (Iron, Copper) accelerate the process. Additionally, highly aerated oils are far more susceptible to Oxidation.
-Thermal: When air bubbles become entrained in the oil, severe failure of the oil may occur due to the conditions known as PID (Pressure-induced Dieseling) Or PTG (Pressure-induced Thermal Degradation). The localized temperatures exceeds 538℃ when air bubbles are collapsed under high pressure, which also lead to thermal degradation.
-Mechanical: “Shearing” occurs when oil molecules are torn apart as they flow between moving mechanical surfaces.
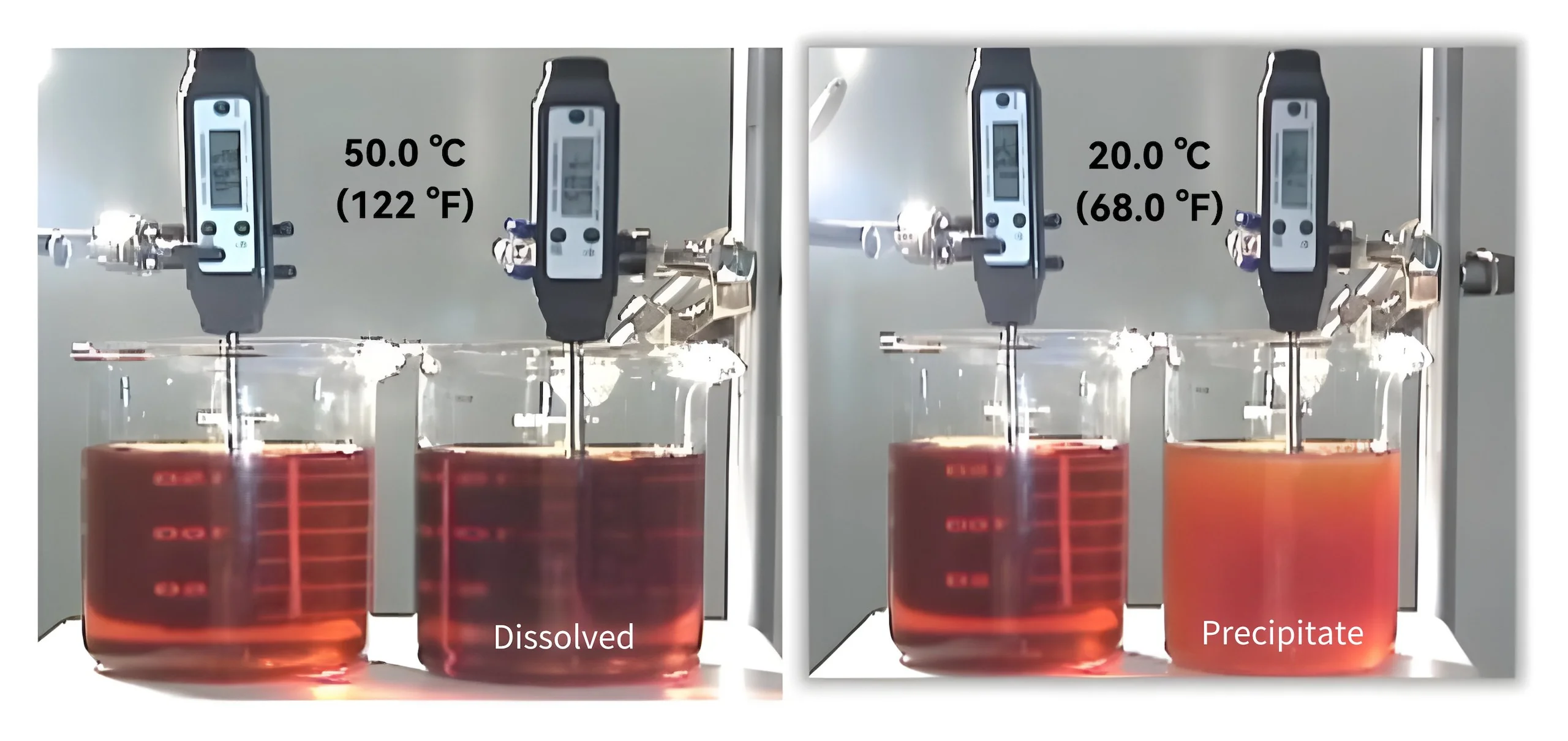
It indicates the capability to dissolve the molecules within the solution that is affected directly by temperature. As by-products of oxidation are continuously created, the fluid is close to saturation point.